ELISA is a plate-based immunoassay used to identify and quantitate antigens, antibodies, and proteins in biological matrices. ELISA uses fluorescent, chromogenic or luminescent techniques to generate detection signals. Since its early inception in the 1960s, It has been routinely used in biomedical and routine diagnoses. Some of the common examples of ELISA are pregnancy tests and the detection of infectious diseases.
Although ELISA has several advantages, immunoassay development and validation are paramount for reliable and robust results. A thoroughly developed method validation ensures it is efficient for the intended purpose. Hence, scientists should always guarantee a rigorous ELISA assay development and validation. So today, we share five tips for a successful assay method.
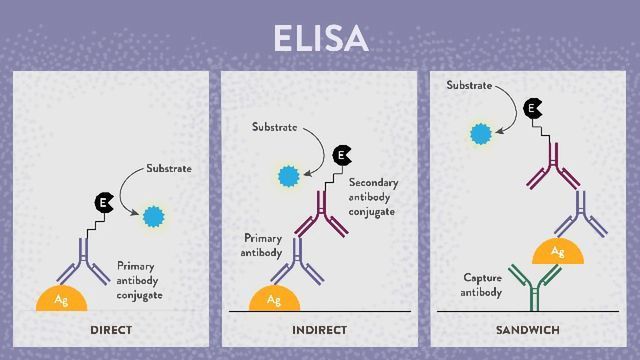
Choosing the best type of ELISA
Researchers and clinicians use ELISA for diagnosing and monitoring disease and drug products. Hence, the type of ELISA to be used depends on the intended goal. Several types of ELISA assays are available for assessing medicine and diseases, including direct, indirect, sandwich, and competitive ELISA. So depending on the intended purpose, the target for an ELISA assay can be an antigen or an antibody. Together with ready-to-use ELISA kits, custom ELISA assay development provides an added edge to assays.
Adequate planning and design
Planning the assay design and workflow can help reduce potential errors and retests. Hence, planning the ELISA assay component ensures the assay run is smooth and efficient. Some important considerations for assay planning include determining the number of assay samples, replicates, and assay plates/kits.
Handling and sample dilution
Incorrect storage or inappropriate handling will affect the assay result. Each assay reagent and buffer has individual storage requirements. For instance, some reagents need to be stored at room temperature, while others might need a freezer. Moreover, if samples are needed to be used for future experiments, then it is ideal to store them in aliquots to avoid unnecessary freeze/thaw cycles.
Maintain accuracy and consistency
Ensuring the workbench is appropriately set up with instruments and components within reach is one of the easiest ways to achieve accuracy and consistency in assay results. Moreover, calibrated precision pipettes reduce the inconsistencies that arise during the pipetting process. Furthermore, scientists must record changes made to an existing ELISA protocol. These records will help other researchers similarly perform the experiment and reduce any performance-associated issues.
Avoid contamination
Contamination is one of the primary reasons for inaccurate ELISA data. Contamination can produce weak or low ELISA signal intensity, high background values, and high well-to-well variation. Following are some simple ways to prevent contamination in ELISA assay development and subsequent analysis.
- Have a clean work environment
- Use distilled or deionized water
- Each transfer should have a clean and new reagent reservoir
- Use new pipette tips for different samples
- Avoid cross-contamination or splashing
- Use clean systems for automated analysis
- Don’t pour reagents back into the stock, as it may cause contamination. Prepare aliquots of the reagents or remove only the required amount for analysis.
Read more blogs – daily time zone